
When you think of natural gas, you may envision something like your gas cooking range or the butane inside a lighter. Even with natural gas being a pivotal part of the U.S. energy sector, the consensus is that most Americans are not too sure what natural gas is or what it does. In this blog, we are going to break down this energy source and explain natural gas (NG), liquified natural gas (LNG), and natural gas liquids (NGLs). From its formation to its application, we’ve got you covered.
 Photo courtesy of iStock/DGLimages
Photo courtesy of iStock/DGLimages
So, what is natural gas and how does it form?
The origin of natural gas lies beneath the earth's surface. Natural gas is a fossil fuel that forms when decomposed animal or plant matter is exposed to large amounts of heat and pressure over many millenniums. The energy originally found in the plant or animal is broken down by heat and pressure then stored in the chemical bonds of natural gases. These natural gas mixtures are primarily composed of methane, ethane, propane, and butane. Some of the more complex alkanes (chemical compounds made of carbon and hydrogen) found in natural gases are isobutane, pentane, and pentanes plus.
What is natural gas used for?
Natural gas benefits many aspects of our daily lives, but its primary use is to generate electrical power. According to the U.S. Energy Information Administration (EIA) “The electric power sector uses natural gas to generate electricity and produce useful thermal output. In 2020, the electric power sector accounted for about 38% of total U.S. natural gas consumption.”
Natural gas is also utilized across other sectors as well including industrial and residential. In the industrial sector, it is used as a raw material or “feedstock” (unprocessed material used to supply a manufacturing process) to produce fuel for heating, fertilizer, and other chemical solutions. In 2020, the industrial sector accounted for 33% of the total U.S. natural gas consumption by sector. Natural gas in the residential sector is used to heat homes/water, to cook, and dry clothes. By 2020, the residential sector accounted for 15% of total natural gas consumption in the United States.
Where is natural gas used?
In 2019, five states accounted for 38% of U.S. natural gas usage:
- Texas: 14.9%
- California: 6.9%
- Louisiana: 6.0%
- Pennsylvania: 5.2%
- Florida: 5.0%
 Photo courtesy of iStock/alvarez
Photo courtesy of iStock/alvarez
Liquefied natural gas. What is it?
Liquefied natural gas is methane that has been cooled to a liquid form of -260°F (-162°C) for transportation and storage. The volume of liquefied methane is approximately 600 times smaller than the volume in its gaseous state.
Why is it liquefied, and what is it used for?
This liquefaction technique, invented in the nineteenth century, allows methane to be transformed to a liquid so it can be transported to remote areas where pipelines do not reach.
Methane, in its compacted liquid state, may also be carried in specialized tankers to ports all over the world. When it arrives at the destination terminal, the liquefied natural gas is then restored to its gaseous state before being carried by pipelines to distribution providers, industrial companies, and power plants.
 Photo courtesy of iStock/JennaWagner
Photo courtesy of iStock/JennaWagner
Natural gas liquids
By definition, natural gas liquids are hydrocarbons in the same family of molecules as natural gas and crude oil, composed only of carbon (C) and hydrogen (H). Natural gas liquids have a wide range of uses in practically every industry such as residential, agriculture, commercial, industrial, transportation, and electrical power sectors.
What are natural gas liquids used for?
Natural gas liquids are used in petrochemical plants, for heating homes, and are even blended into vehicle fuel. Types of natural gas liquids include ethane, propane, and butane.
Types of natural gas liquids and where they are used
Methane CH4:
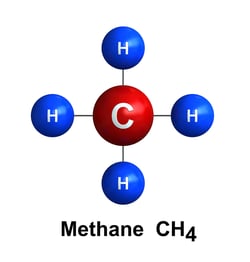 Photo courtesy of iStock/oorka
Photo courtesy of iStock/oorka
Methane is primarily used for heating and lighting homes. However, it can also be used as an energy source for water heaters, cooking, and fireplaces, and as a feedstock for organic chemicals.
Ethane C2H6:
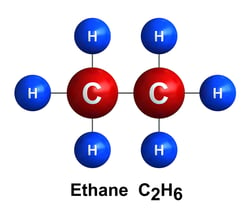 Photo courtesy of iStock/oorka
Photo courtesy of iStock/oorka
Ethane is used as a petrochemical feedstock to produce ethylene (a hydrocarbon group C2H4 extracted from ethane), which is then used to make plastics. It is also used to make antifreeze, detergent, and other chemical solutions.
Propane C3H8:
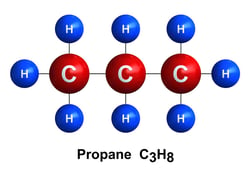 Photo courtesy of iStock/oorka
Photo courtesy of iStock/oorka
Propane may also be used as a petrochemical feedstock to produce ethylene and propylene. When utilized for energy, it is often used in households to power stoves, gas grills, clothes dryers, generators, and water heaters. It can also be utilized as a fuel to power cars, delivery vans, buses, lawnmowers, and forklifts.
Butane C4H10:
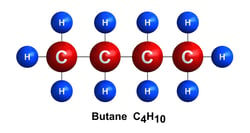 Photo courtesy of iStock/oorka
Photo courtesy of iStock/oorka
Butane is a common petrochemical feedstock used in the production of synthetic rubber. It can also be used to produce lighter fluid or as a component in gasoline blending.
Connecting the dots
A lot was just unpacked, so let’s piece it all together. The versatile organic matter of natural gas is the origin of it all. Natural gas mixtures are primarily composed of methane, ethane, propane, butane, isobutane, pentane, and pentanes plus. Liquefied natural gas is used as a transportation method for methane. It is cooled to low temperatures to reduce its volume, allowing it to be stored and shipped in specialized tanks to remote regions of the world. Natural gas liquids are used in different hydrocarbon molecular formulas to power and create products in the residential, agriculture, commercial, industrial, transportation, and electrical power sectors.
This is the first of a two-part blog on natural gas. Check back soon to read about Dixon’s role in the liquefied natural gas sector. If you enjoyed this blog post, please share it with your colleagues!
Source: U.S. Energy Information Administration, Natural Gas Annual, September 2020


